Gene Regulation Contributing to Cancer and HIV Pathogenesis
Normal cell growth and development are highly regulated by hormones and factors from the environment, and cells respond to these signals by regulating expression of appropriate genes. Many diseases are caused or influenced by alterations in the relationship between cell signaling and gene regulation. For example, development of cancer can largely be attributed to uncoupling of gene regulation from growth factor signaling. Similarly, the pathology of AIDS is strongly influenced by control of HIV-1 gene expression through T cell activation signals. To elucidate fundamental mechanisms controlling signal responsive expression, we study the regulation of specific transcription factors in human T cells and the model eukaryotic organism Saccharomyces cerevisiae . Our research has been instrumental in revealing how responses to multiple signals can be coordinated through the function of the RNA polymerase II-associated protein kinase CDK8. Additionally, we have identified a human transcription factor that regulates establishment of latent HIV-1 provirus and controls reactivation of viral replication in response to T cell signaling.
Regulation of HIV transcription by T cell signaling
HIV-1 gene expression is tightly linked to T cell activation, and this relationship contributes to pathology of AIDS. During the course of infection, a population of latently infected resting memory T cells develop which have transcriptionally repressed chromosomally integrated copies of the HIV genome. These latently infected cells contribute to disease progression by acting as reservoir of inducible virus production throughout the course of infection. Furthermore, latent provirus is unaffected by current AIDS drugs, and without new therapies that target this population, the virus can never be eliminated from infected individuals.
Expression of viral genes is controlled by the 5′ Long Terminal Repeat (LTR) promoter, which has binding sites for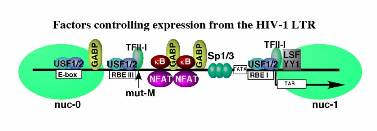 numerous transcription factors present in T cells (Figure 1). Our research has identified a factor termed RBF-2, which binds at two places on the viral promoter. In unstimulated cells RBF-2 contributes to repression of transcription, but in stimulated cells this factor is required for induction in response to signals from the T-cell receptor. We have purified RBF-2 from T cell nuclear extracts and determined it to be comprised of a complex of proteins consisting of the factors USF1, USF2 and TFII-I. Specific interaction between TFII-I and USF1 promotes their binding to RBEIII (Figure 2), and this interaction is essential for induction of chromosomally integrated latent provirus. We have proposed that RBF-2 regulates the establishment of latency and re-induction in response to T cell signaling by controlling chromatin organization on the LTR, which in turn influences access of additional factors with the viral enhancer region. Considering the importance of RBF-2 (TFII-I/ USF1/ USF2) for regulation of the integrated provirus, we are currently using novel protein interaction technology developed in our lab to identify specific inhibitors of the USF1-TFII-I interaction, with the expectation that these will be useful in treating the latently infected memory T cell population. In our ongoing experiments, we are examining the molecular mechanisms that control RBF-2 in response to T cell signaling, and how these factors bound at RBEI and RBEIII regulate chromatin organization and expression of the integrated virus.
numerous transcription factors present in T cells (Figure 1). Our research has identified a factor termed RBF-2, which binds at two places on the viral promoter. In unstimulated cells RBF-2 contributes to repression of transcription, but in stimulated cells this factor is required for induction in response to signals from the T-cell receptor. We have purified RBF-2 from T cell nuclear extracts and determined it to be comprised of a complex of proteins consisting of the factors USF1, USF2 and TFII-I. Specific interaction between TFII-I and USF1 promotes their binding to RBEIII (Figure 2), and this interaction is essential for induction of chromosomally integrated latent provirus. We have proposed that RBF-2 regulates the establishment of latency and re-induction in response to T cell signaling by controlling chromatin organization on the LTR, which in turn influences access of additional factors with the viral enhancer region. Considering the importance of RBF-2 (TFII-I/ USF1/ USF2) for regulation of the integrated provirus, we are currently using novel protein interaction technology developed in our lab to identify specific inhibitors of the USF1-TFII-I interaction, with the expectation that these will be useful in treating the latently infected memory T cell population. In our ongoing experiments, we are examining the molecular mechanisms that control RBF-2 in response to T cell signaling, and how these factors bound at RBEI and RBEIII regulate chromatin organization and expression of the integrated virus.
Regulation of signal responsive gene expression in yeast
Most of our current knowledge of the basic mechanisms for eukaryotic gene expression have come from experiments with model organisms such as the budding yeast Saccharomyces cerevisiae . We are studying GAL4 and STE12, two transcriptional activators that control distinct sets of genes in response to nutrient availability and mating pheromone. Our research examining phosphorylation of these factors resulted in identification of a function for CDK8, a protein kinase associated with RNA polymerase II. We have shown that CDK8 activity is controlled by general physiological signals, including nutrient availability and stress conditions to which the cell must mount global alterations in gene expression to enable a proper growth response. Our results show that interaction between CDK8 and specific transcription factors allows for coordination of such global signals with responses to specific signals that regulate smaller subsets of genes.
A good illustration of this relationship is provided by the factor GAL4, which regulates expression of genes required for utilization of galactose (Figure 3). GAL4 is an important model for eukaryotic gene regulation because it is subject to control by both positive and negative signals; activity is induced by the presence of galactose, but is inhibited by glucose. GAL4 is phosphorylated during transcriptional activation by both CDK7 and CDK8 of the general transcription factor machinery. CDK8 phosphorylates GAL4 on serine 699, and this modification is necessary for full induction by galactose. Because CDK8 activity is inhibited by nutritional or physiological stresses, we have proposed that S699 phosphorylation functions as a “throttle” for the GAL genes to modulate the activity of GAL4 to levels that are appropriate for the growth environment.
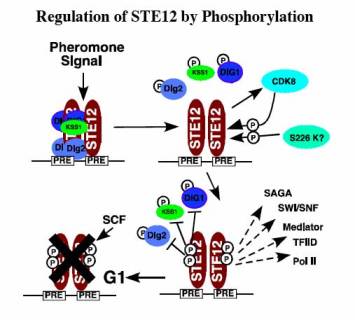
STE12 activates multiple distinct classes of genes in response to different signals. In haploid yeast, STE12 is required for activating genes required for response to peptide mating pheromones produced by the opposite mating type. The signal transduction pathway controlling mating pheromone response contains a prototypical MAP kinase cascade, and is similar to growth signal transduction pathways of higher eukaryotes. STE12 activity is regulated by this signaling pathway through interaction with the pheromone-responsive MAP kinases, and the DIG1 and DIG2 inhibitor proteins. STE12 becomes phosphorylated on several residues by CDK8 during transcriptional activation, but unlike GAL4, these phosphorylations target STE12 for degradation by a ubiquitin-proteasome-dependent mechanism during G1 phase of the cell cycle (Figure 4). CDK8 activity is inhibited under conditions of nutrient starvation, and we have shown this allows accumulation of STE12 protein for activation of filamentous response genes, a morphological differentiation adapted by yeast to cope with nutrient deficiency.
Our current research on these factors is focused on elucidating molecular details of how their phosphorylations and interactions with regulatory proteins control activity in response to specific signaling. Additionally, because both factors are controlled by CDK8, we are using a combination of genetic and biochemical approaches towards identification of the signaling pathways that control CDK8 activity in response to nutritional and physiological cues. These projects will continue to provide valuable insight into fundamental mechanisms for regulation of eukaryotic gene expression by signal transduction pathways.